ASBTDC client Rejuvenix Technologies has won a $383,213 National Cancer Institute Small Business Innovation Research Phase I contract to develop encapsulated radiation-triggered liposomes intended to achieve safer, controlled delivery of chemotherapy.
This technology will be used to treat non-metastatic lung cancer, overcome systemic toxicity, and improve clinical outcomes for millions of cancer patients.
The company is teaming with the University of Arkansas for Medical Sciences on the project.
“Our patented technology utilizes localized ionizing radiation to trigger the release of chemotherapeutic agents that are contained within radio-sensitive liposomes. The technology greatly minimizes the toxic exposure to vital organs while potentially enhances overall efficacy of combined radiation and chemotherapy standards of care,” said Joshua Phillips, CEO of Rejuvenix.
“For example, when you eliminate weeds in your lawn, you spray just the weeds and not the entire lawn. Similarly, radiation is the activator of the drug delivery which is a treatment that is precisely targeted only into the three-dimensional tumor mass. This triggers the release of the chemotherapy formulations developed by Rejuvenix selectively within the tumor where the radiation exposure occurs.”
ASBTDC Assistance
ASBTDC supported Rejuvenix through the SBIR award process with timely market research and the expert knowledge and proposal reviewing skills of Innovation Specialist Rebecca Todd.
“Our work with Rebecca Todd at ASBTDC was critical to our success in winning this SBIR contract. Rebecca assisted with reviews of content that we integrated into our application and provided ‘just-in-time’ guidance to support our response to requests for additional information prior to the award decision. Through her efforts at ASBTDC, we received a comprehensive report on the market landscape related to our platform,” said Phillips.
The Team
The Rejuvenix team includes Phillips, Dr. Amanda Stolarz as chief scientific officer, Dr. Jay Gandy as chief regulatory officer, and Robert Griffin, PhD as principal investigator and senior vice president for research and development.
Phillips believes Rejuvenix is in a strong position to commercialize the technology, due to the team’s expertise.
“The experience of our founders includes business startup and development, FDA approval expertise, pharmaceutical testing, radiation and biophysics, and nano synthesis of liposomes. Alongside this broad experience is our commitment to an easily remembered goal to improve patient outcomes by limiting the toxic side effects of chemotherapy.
“In addition to our internal expertise, we are working with our connections to other leading research institutions to quickly test and validate our formulations,” he said.
The Need
Acute chemotherapy toxicity is a driving factor in 30-40% of patients switching, interrupting, or stopping therapy, limiting the effective dose that can be administered to the patient and ultimately leading to decreased overall cancer survival.
“Current treatments that involve chemotherapeutics have many adverse side effects and contain a set of well-known cancer-killing drugs that expose a patient’s entire body to the treatment, similar to our analogy to spraying your entire lawn with a weed killer,” said Griffin.
The management of symptoms related to chemotherapy toxicity is costly and time-consuming, plus it significantly decreases quality of life for the patient. Despite these issues, chemotherapy combined with radiotherapy continues to be one of the only options to generate at least a measurable significant clinical benefit.
The Rejuvenix team worked through the Delta I-Fund to validate the opportunity associated with its platform. “We interviewed over 75 doctors, patients, scientists, investors, and regulators to evaluate the technology. We also worked with the ASBTDC to complete market analysis of current alternatives and their value in the marketplace,” Stolarz said.
Solution
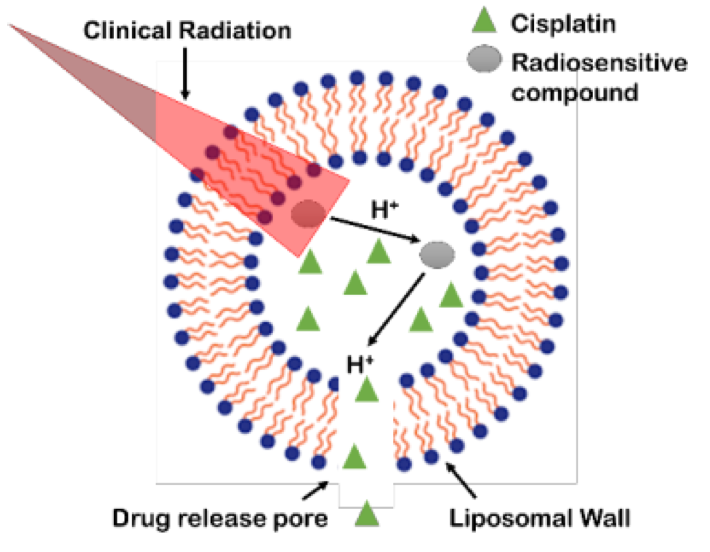
Rejuvenix’s methodology targets both radiotherapy and the delivery of chemotherapeutics to solid tumors. Through its platform, chemotherapy can be encapsulated to prevent it from exposing off-target sites in the body, and trigger its release once the encapsulated drug is in the tumor.
“Our platform will eliminate or significantly reduce the impact of chemotherapy side effects to other vital organs. It also presents the opportunity for chemotherapy to damage or kill tumor cells very near the time that radiation damages the cells, which we expect will create a more than additive total amount of tumor cell killing while reducing the typical systemic effects of traditional chemotherapy, which include a weakened immune system and potential for life threatening infections among other damage to normal tissues,” said Griffin.
The Phase I SBIR Project
“At the end of our project we will have a better understanding of how well the drug works once administered and what, if any, side effects or offsite release could occur,” said Griffin.
Griffin, a professor in Radiation Oncology at UAMS, is leading a team of Rejuvenix and UAMS scientists to complete the validation.
Rejuvenix has received an additional $50,000 in SBIR matching funds from the Arkansas Economic Development Corporation.
“Together these funds are providing the capital we need to complete significant formulation and testing milestones, in addition to further de-risking the platform through proof of concept testing,” said Phillips.
What’s Next
“Rejuvenix is focused upon successfully completing our SBIR Phase I contract. We are seeking to join the cohort for the 2021 I-Corps to further develop our commercialization plan alongside the Phase I contract. At the conclusion of our Phase I contract we will begin seeking angel investors alongside our summer 2021 efforts to secure a Phase II SBIR to further our commercialization efforts,” Phillips said.
This project has been funded in whole or in part with Federal funds from the National Cancer Institute, National Institutes of Health, Department of Health and Human Services, under Contract No. 75N91020C00054